Abstract
Recently, there is significant increase in globalisation in industrial areas especially in developed countries. Thus, their waste by-products become a major challenge because of the large quantities involved and the scarcity of disposal space. Industrial waste by-products and the use of traditional cementitious binders (e.g. lime, cement, and gypsum) in geotechnical applications are facing many challenges due to high greenhouse gas emissions, intensive use of energy and depletion of natural resources. In addition, most of the traditional binders are not readily acceptable due to stringent occupational health and safety issues as well as threats to the soil and groundwater environment. To address these challenges, new binders using industrial waste-by products are being investigated. Among these, alkaline activation is one of the most interesting methods being developed; it has equal or better performance than cement-based binders, but with lower environmental issues and costs. This paper reviews the key developments in alkali-activated materials since 2011, with a particular focus on advances in characterisation techniques, structural understanding, binder precursors, activation approaches, design and processing, and reaction mechanism. Finally, the paper proposes further research and development topics and suggests steps forward to enhance the potential application of these materials for ground improvement.
1. INTRODUCTION
Loss of soil stiffness and strength due to liquefaction and consequent large ground deformation has caused extensive structural damage and economic losses in urban areas during major earthquakes. Therefore, wide ranges of remedial measures have been developed for treating or improving soils against liquefaction. These include densification, solidification, pore pressure relief, lowering ground water table, and restraint of shear deformation. Moreover, cementitious materials like cement have been widely utilised in different mitigation techniques such as compacting grouting, deep soil mixing, and jet grouting (Srbulov, 2009). These chemical admixtures are often used as an additive to improve the strength and stiffness of soils (Tatsuoka et al., 1995). However, the improvement in shear strength using traditional additives is often associated with decreased plasticity and enhanced brittleness attributed to pozzolanic hardening. Cement industry not only accounts for around 5-7% of global carbon dioxide emissions and environmental footprint such as global warming and soil ecotoxicity, but also has setting time challenge. One potential replacement for cementitious materials is alkali-activated materials (AAMs) which seem to yield similar mechanical properties like cementitious materials (Provis, 2014). Alkali activation is the generic term which is applied to the reaction of a solid aluminosilicate (termed the ‘precursor’) under alkaline conditions (induced by the ‘alkali activator’), to produce a hardened binder, which is based on a combination of hydrous alkali-aluminosilicate and/or alkali-alkali earth-aluminosilicate phases (Provis et al., 2015). Additional terminology often used to refer to these materials includes ‘geopolymer’ nomenclature that is used largely to describe low-calcium alkali-activated aluminosilicate binders. The defining characteristic of a geopolymer is that the binding phase comprises an alkali aluminosilicate gel, with aluminium and silicon linked in a three-dimensional tetrahedral gel framework that is relatively resistant to dissolution in water (Palomo et al., 2014). According to a recent definition by Provis (2014), alkali activated materials are produced through the reaction of an aluminosilicate, normally supplied in powder form as an industrial by-product or other inexpensive materials, with an alkaline activator which is usually a concentrated aqueous solution of alkali hydroxide, silicate, carbonate or sulphate. In recent years, geopolymer has attracted considerable attention among these binders because of its compressive strength, low permeability, good chemical resistance and excellent fire resistance behaviour (Provis, 2017). Because of these advantageous properties, geopolymer is a promising alternative to cementitious materials in addressing various geotechnical problems and waste immobilization solutions for the industries. The aim of the current paper is to review and highlight the key recent scientific enhancements in the development, characterisation, processing and environmental assessment of AAMs.
2. ALKALINE ACTIVATION AND GEOTECHNICAL APPLICATIONS
It is noticeable that using alkaline activation in geotechnical applications is still at the early stage of development. However, Table 1 summarises the existing literature about utilising alkali-activated binder for the purpose of ground improvement. In very limited attempts, some geotechnical researchers investigated the effectiveness of precursors, including fly ash (FA), metakaolin, blast furnace slag (GGBS), palm oil fuel ash (POFA) and red gypsum (RG), with soft soil in the presence of a predesigned concentrated aqueous alkaline hydroxide or silicate solution. In this respect, Cristelo et al. (2013) address the effectiveness of alkali-activated low-calcium and high-calcium FA as silica and alumina amorphous sources. Based mostly on the microstructural analysis, these authors demonstrated that a binding gel (either N–A–S–H and/or C–A–S–H) is developed inside the soil voids, helping to form more compact microstructures and, as a consequence, improved compressive strength. Moreover, the authors reported that the short-term strength gain of the stabilised soil is faster when high-calcium FA was used as a precursor. Sargent et al. (2013) carried out a research work to study the feasibility of using some alkali-activated by-products, such as GGBS, FA and RG, on some geotechnical properties of soft soil. Based on the test results, they concluded that alkali-activated GGBS, GGBS–FA and GGBS–RG exhibited significant strength regarding the untreated soil. Pourakbar et al. (2015) reported that the addition of highly alkaline solutes, including NaOH and KOH, increased the strength of the treated soil samples. According to this study, curing time and the water content of soil were also shown to have a significant strengthening effect on the treated soil.
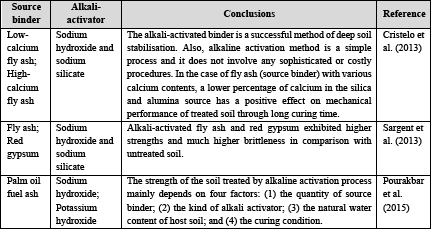
Table 1: Summary of alkali-activated binders for ground improvement
3 ADVANCES IN CHARACTERISATION
3.1 Nanostructural Characterisation
The nanostructure of AAMs is strongly dependent on the available calcium content of precursors; a high-calcium system such as alkali-activated blast furnace slag is dominated by a calcium aluminosilicate hydrate (C–A–S–H) gel with a to bermorite-like structure (Provis et al., 2014), while low-calcium systems such as those based on meta-kaolin or fly ash tend to generate an alkali aluminosilicate (N–A–S–H) gel with a highly cross-linked, disordered pseudo-zeolitic structure (Richardson et al., 1994). These gels can coexist in binders based on blends of high-calcium and low-calcium precursors (Davidovits, 2005; Provis, 2014). Fourier transform infrared (FTIR) spectroscopy is a key technique for the analysis of AAMs, particularly for low-calcium systems where it can probe the connectivity within Si–O– (Si, Al) frameworks via shifts in the peak corresponding to the asymmetric stretch of that bond (Provis, 2014). The gel theory of an initial Al-rich binder gel (“Gel 1”) forming at the first hours, and then evolving to a more Si-rich structure (“Gel 2”), which was developed from ex-situ analysis of the gel evolution (Provis et al., 2015) has been refined. Note that FTIR spectroscopy (see Figure 1) detects bond vibrations rather than the actual nuclei. The ‘Gel 1’ stage involves a high degree of formation of Si–O–Al bonds relative to the bulk Si/Al ratio, because the formation of cross-links involving Al atoms joining between Si sites is rapid. This leads to a gel, which has a relatively high concentration of Si–O–Al bonds (Richardson et al., 1994).